An experiment measured the cathodic current for five solutions of known 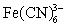 concentration using cyclic voltammetry.The collected data are plotted and the best line fit applied.If an unknown ferricyanide solution has a cathodic current of 17.38 A,calculate the concentration ferricyanide in the unknown.
concentration using cyclic voltammetry.The collected data are plotted and the best line fit applied.If an unknown ferricyanide solution has a cathodic current of 17.38 A,calculate the concentration ferricyanide in the unknown. 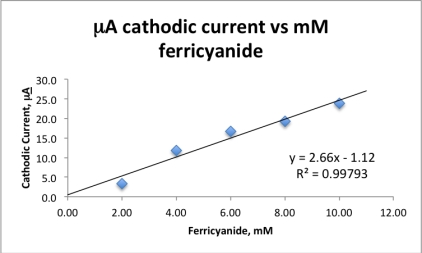
Correct Answer:
Verified
Q4: For a three-electrode cell the working electrode
Q5: When a potential is applied to a
Q6: Which are true for polarography?
I Polarography is
Q7: The_ is the electrode at which the
Q8: Which statement(s)is(are)defined INCORRECTLY?
I Ohmic potential is the
Q10: Which is NOT true of overpotential?
A)Overpotential is
Q11: A technician is tasked with testing the
Q12: _ is a set of techniques that
Q13: Which is NOT true for square wave
Q14: Sulfide in a water sample is titrated
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents