To Measure Calcium in a Breakfast Cereal,0 G/mL)is Added,diluting to the Final Volume with Distilled Deionized H2O
To measure calcium in a breakfast cereal,0.6590 grams of crushed cereal were reacted for 1 hour in warm 1 M HNO3.The reaction mixture was filtered and the filtrate transferred to a 100 mL volumetric flask.A series of eight standards are prepared by transferring 5.00 mL aliquots to 50-mL volumetric flasks to which increasing volumes of standard Ca2+ (containing 20.0 g/mL)is added,diluting to the final volume with distilled deionized H2O.The samples were then analyzed by flame atomic absorption.Use the data and graph below to find the percent by mass calcium in the cereal.
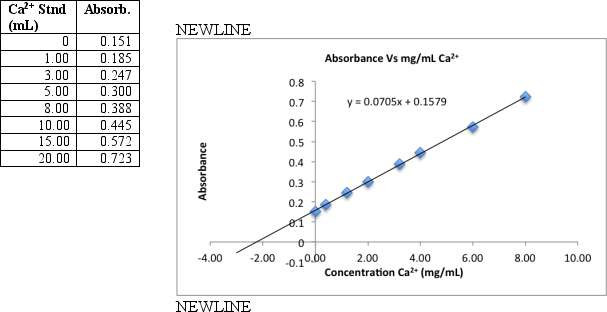
Correct Answer:
Verified
Q1: All of the following are true for
Q2: X-rays are generated when:
A)metals such as W,Mo,Ag,or
Q3: Which is NOT true of the flame
Q4: Which of the following statements are true
Q6: The dynamic reaction cell reduces isobaric interference
Q7: Graphite furnace employs temperature programming to produce
Q8: There is no universal flame temperature for
Q9: Inductively coupled argon plasma does not suffer
Q10: _ is any effect that changes the
Q11: Which of the following statements are true
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents