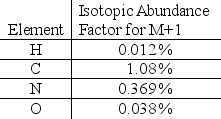
The mass spectrum of an unknown compound has a molecular ion peak at 194 m/z.The proposed chemical formula for the compound is C8H10N4O2.The M+1 peak has a relative abundance of 10.1%.Does the intensity of the M+1 peak support the proposed chemical formula?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q7: Calculate the number of rings and bonds
Q8: When identifying the molecular ion peak,
Q9: The three essential components of any mass
Q10: Which statements are true for the various
Q11: Analytes must be ionized prior to entering
Q13: Electrospray ionization is one of many techniques
Q14: Which is NOT true for ion mobility
Q15: Calculate the number of rings and double
Q16: Ion mobility spectrometry_and_ .
A)operates at ambient pressure;is
Q17: Which is NOT true of mass spectrometers?
A)Double-focusing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents