Faraday's Law of Electrolysis states that the mass of a substance produced at an electrode during electrolysis (m) is directly proportional to the number of moles of electrons transferred at that electrode (n) .Which of the following equations correctly characterizes the relation?
A) m = k × n
B) As the number of electrons transferred increases the mass deposited decreases.
C) There is one conversion factor possible that relates m and n.
D) 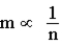
E) All of the above correctly describe this relation.
Correct Answer:
Verified
Q23: Consider the three thermometers shown in the
Q24: A solution is prepared by adding 1.77
Q25: The meter stick in the image is
Q26: Which of the following is the best
Q27: How many significant figures are in each
Q29: Acetone boils at 56 °C.Express this temperature
Q30: Consider the following measured number.640,000 m If
Q31: A filled shipping box weighs 12 lb.How
Q32: The melting point of iron is 1535.0°C.What
Q33: The temperature in the laboratory is posted
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents