Choose the correct equilibrium constant expression for the equilibrium
4 NO(g) + 6 H2O(g)  4 NH3(g) + 5 O2(g)
4 NH3(g) + 5 O2(g)
A) K = 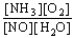
B) K = 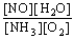
C) K = 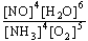
D) K = 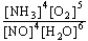
E) K = 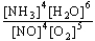
Correct Answer:
Verified
Q16: Which of the following is the primary
Q17: Consider the following reaction coordinate diagram.
Q18: How does increasing the temperature increase the
Q19: Consider the hypothetical reaction A + 2
Q20: Which of the following statements is/are incorrect?
i.Undissolved
Q22: Choose the correct equilibrium constant expression for
Q23: If a copper(II)nitrate solution is added to
Q24: Predict what will happen if the partial
Q25: Consider the hypothetical reaction A2 + B2
Q26: If the position of equilibrium shifts to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents