If an aluminum nitrate solution is added to a sodium fluoride solution the following equilibrium will be reached: Al3+(aq) + 6 F-(aq)  AlF63-(aq) .For this equilibrium,K = 5.0 × 1023.Choose the correct equilibrium constant and the correct direction favored at equilibrium.
AlF63-(aq) .For this equilibrium,K = 5.0 × 1023.Choose the correct equilibrium constant and the correct direction favored at equilibrium.
A) K = 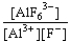 reverse
reverse
B) K = 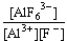 forward
forward
C) K = 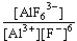 reverse
reverse
D) K = 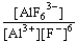 forward
forward
E) K = 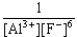 forward
forward
Correct Answer:
Verified
Q34: If one is trying to change the
Q35: K = 1.3 × 10-10 for the
Q36: Calculate the molar solubility of aluminum phosphate
Q37: What will happen to the position of
Q38: Which of the following statements is/are correct?
i.If
Q40: What will happen if the temperature is
Q41: Which of the following correctly expresses Ka
Q42: Ka = 1.8 × 10-5 for the
Q43: Find the pH of a 0.10 M
Q44: What is the pH of a 0.20
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents