Consider the following image which depicts the reactants in an acid-base reaction.Atoms other than H are labeled with the element symbol. 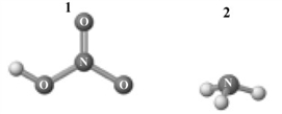 The products of this reaction are shown below.
The products of this reaction are shown below. 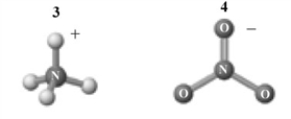 Which of the following is a correct interpretation of this reaction?
Which of the following is a correct interpretation of this reaction?
A) 1 and 2 are a conjugate acid-base pair.
B) 3 and 4are a conjugate acid-base pair.
C) 1 and 4 are a conjugate acid-base pair.
D) 2 and 4 are a conjugate acid-base pair.
E) 1 and 3 are a conjugate acid-base pair.
Correct Answer:
Verified
Q11: Which of the following conforms to the
Q12: Which of the following is not capable
Q13: According to Arrhenius theory,what is an acid?
A)A
Q14: Which of the following is a characteristic
Q15: Which of these statements is true?
A)All Brønsted-Lowry
Q17: Which of the following cannot act as
Q18: What is the conjugate base of water?
A)HCl(aq)
B)H3O+(aq)
C)OH-(aq)
D)H+
Q19: Which of the following substances is amphoteric?
A)H2O(
Q20: A substance that can act as both
Q21: Given the following acid strengths: HF >
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents