The cylinder shown contains 0.79 moles of nitrogen,0.19 moles of oxygen and 0.02 moles carbon dioxide,a total of 1.00 mole of molecules in the approximate proportion in which they are present in air.Of the three gases,only carbon dioxide is appreciably soluble in the water in the well at the bottom.Assume an equilibrium between dissolved and undissolved carbon dioxide at the beginning and sufficient time lapse to reestablish that equilibrium after the change described. 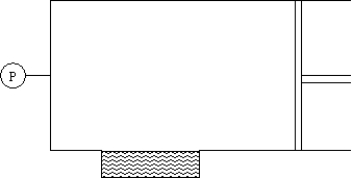 If 0.02 mole of carbon dioxide is forced into the cylinder,the solubility of carbon dioxide:
If 0.02 mole of carbon dioxide is forced into the cylinder,the solubility of carbon dioxide:
A) increases by a factor of about 50
B) increases by a factor of about 2
C) increases by 2%
D) remains unchanged
E) decreases
Correct Answer:
Verified
Q12: Consider the graph shown below. 
Q13: Which of the following statements is incorrect?
A)If
Q14: What is the molality of a solution
Q15: The structures of cyclohexane and benzene are
Q16: Consider the graph shown below. 
Q18: You are required to prepare a 3.50%
Q19: If 45.5 g of BaCl2 is dissolved
Q20: Which of the following statements is incorrect?
A)The
Q21: What volume of water,which has a density
Q22: 75.0 mL of water is added to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents