The graph below represents a temperature versus energy plot for a pure substance. 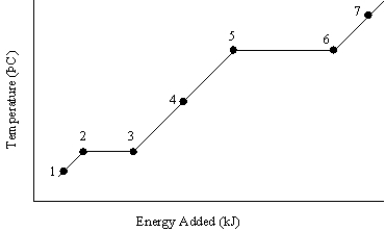 Identify the point(s) where:
Identify the point(s) where:
(i) only gas exists
(ii) both solid and liquid exist
A) (i) 7 (ii) 2 and 3
B) (i) 7 (ii) 5 and 6
C) (i) 1 (ii) 2 and 3
D) (i) 4 (ii) 5 and 6
E) (i) 4 (ii) 2 and 3
Correct Answer:
Verified
Q28: The specific heat of solid gold is
Q29: Why does ice float on liquid water?
A)It
Q30: Which of the following statements is correct?
A)There
Q31: How are network solids,such as diamond,C,and quartz,SiO2,distinguished
Q32: Which of the following statements about change
Q34: A sample of lead releases 221 kJ
Q35: Among the following,identify the incorrect statement about
Q36: What quantity of water will form by
Q37: Calculate the specific heat of an unknown
Q38: Closed system A consists of liquid acetone
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents