Consider the following representation of a gaseous chemical reaction. 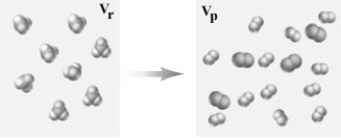 If carried out at constant temperature and pressure how would the volume in the container before the reaction (Vr) compare to that after the reaction (Vp) ?
If carried out at constant temperature and pressure how would the volume in the container before the reaction (Vr) compare to that after the reaction (Vp) ?
A) Vr = Vp
B) Vr = 2Vp
C) 2Vr = Vp
D) 3Vr = Vp
E) Vr = 3Vp
Correct Answer:
Verified
Q2: An unknown substance is vaporized at 200°C
Q3: Calculate the density of nitrogen dioxide at
Q4: Which of the following is a mathematical
Q5: What is the density of helium gas
Q6: Calculate the density of neon at 32°C
Q8: Calculate the number of moles in 3.13
Q9: Which of the following gases should have
Q10: Consider the balloon shown below which has
Q11: For which state of matter represented in
Q12: Determine the density of nitrogen gas at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents