If 10.0 g of aluminum is consumed in the reaction 2 NaOH(aq) + 2 Al(s) + 6 H2O( 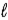 ) → 2 NaAl(OH) 4(aq) + 3 H2(g) ,what volume of hydrogen,measured at -4°C and 0.88 atm,will be produced?
) → 2 NaAl(OH) 4(aq) + 3 H2(g) ,what volume of hydrogen,measured at -4°C and 0.88 atm,will be produced?
A) 6.2 L
B) 12 L
C) 14 L
D) 16 L
E) 19 L
Correct Answer:
Verified
Q37: How many liters of hydrogen,measured at 20°C
Q38: Calculate the volume of 76.2 mole of
Q39: Which of the following statements is incorrect?
i.The
Q40: What is the molar volume of CO2
Q41: An STP volume of 564 liters of
Q42: If 26.0 liters of methane,CH4,measured at 1.09
Q43: Consider the reaction CO(g)+ 2 H2(g)→ CH3OH(g).If
Q45: 37.9 liters of NO at 725 torr
Q46: Liquid butane burns according to the equation
Q47: A 399 mL sample of ethane at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents