Which element will act as the negative pole in each of the following bonds: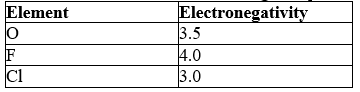
A) F in O-F and O in O-Cl
B) O in O-F and O in O-Cl
C) F in O-F and Cl in O-Cl
D) O in O-F and Cl in O-Cl
E) O, F, and Cl are all highly electronegative so there will be neither a positive nor a negative pole.
Correct Answer:
Verified
Q34: How many single,double,and triple bonds are in
Q35: Which of the following is the best
Q36: How many electrons are in a single,double,and
Q37: Which of the following statements is incorrect?
A)Electronegativities
Q38: Arrange the following bonds in order of
Q40: Which of the following terms best describes
Q41: Which of the following is the best
Q42: If you were to sketch a particulate-level
Q43: Match each of the following terms with
Q44: Which of the following correctly describes the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents