Using the graph below,determine the gas that has the lowest density at STP. 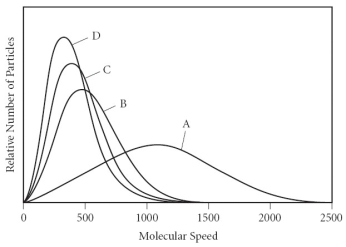
A) A
B) B
C) C
D) D
E) All of the gases have the same density at STP.
Correct Answer:
Verified
Q27: What is the mole fraction of CO
Q28: Which of the following samples will have
Q29: Which of the following samples will have
Q30: A mixture of N2,O2 and Ar has
Q30: Determine the density of NH3 gas at
Q32: What volume will 0.780 moles of He
Q33: A mixture of 1.0 mol He and
Q34: A mixture of 0.220 moles CO,0.350 moles
Q35: The density of a gas is 1.43
Q36: Which of the following samples has the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents