The specific heat capacity of liquid water is 4.18 J/g ∙ K.How many joules of heat are needed to raise the temperature of 5.00 g of water from 25.1°C to 65.3°C?
A) 48.1 J
B) 840 J
C) 1.89 × 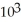
J
D) 2.08 × 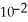
J
E) 54.4 J
Correct Answer:
Verified
Q42: Use the ΔH°f and ΔH°rxn information provided
Q58: Choose the thermochemical equation that illustrates ΔH°f
Q60: Match the following.
--ΔE
A)work done by the system
Q61: Explain the difference between ΔH and ΔE.
Q64: Calculate the kinetic energy of a 150
Q65: Give the equation to calculate the enthalpy
Q107: Calculate the work,w,gained or lost by the
Q114: For a process at constant pressure,49,600 calories
Q129: A 5.00-g sample of liquid water at
Q139: A 6.50-g sample of copper metal at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents