The enthalpy change for converting 10.0 g of ice at -25.0°C to water at 80.0°C is ________ kJ.The specific heats of ice,water,and steam are 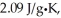
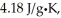 And
And 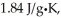 Respectively.For H2O,∆Hfus = 6.01 kJ/mol,and ∆Hvap = 40.67 kJ/mol.
Respectively.For H2O,∆Hfus = 6.01 kJ/mol,and ∆Hvap = 40.67 kJ/mol.
A) 12.28
B) 6.16
C) 3870
D) 7.21
E) 9.88
Correct Answer:
Verified
Q95: Q98: The normal boiling point for H2Se is Q99: Q102: How many atoms are present in a Q103: Identify the compound that does not have Q104: In the phase diagram,what is boiling point? Q105: What fraction of an atom is at Q118: In liquid propanol,CH3CH2CH2OH Which intermolecular Q151: Cesium has a radius of 272 pm Q152: How much heat is released when 105![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents