Which of the following represents the integrated rate law for a second-order reaction?
A) 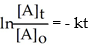
B) 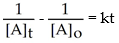
C) 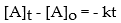
D) k = Ae(-Ea/RT)
E) 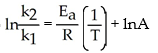
Correct Answer:
Verified
Q28: What data should be plotted to show
Q29: The first-order decomposition of cyclopropane has a
Q31: Determine the rate law and the value
Q32: Given the following rate law,how does the
Q33: Determine the rate law and the value
Q35: Which of the following represents the integrated
Q37: Which of the following represents the equation
Q38: Determine the rate law and the value
Q39: Given the following rate law,how does the
Q40: Determine the rate law and the value
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents