The graph represents the first-order decomposition of N2O5(g) to form NO2(g) and O2(g) .
N2O5(g) → 2NO2(g) +1/2O2(g)
What is the estimated rate constant?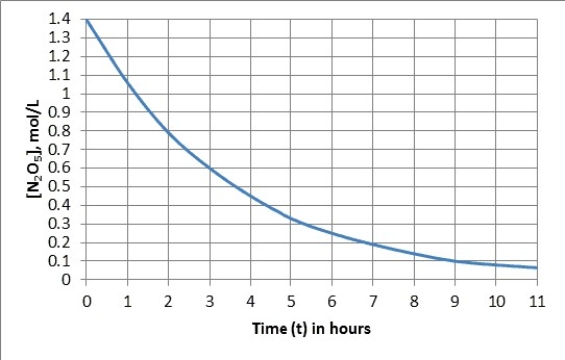
A) 0.28 hr-1
B) 0.70 hr-1
C) 0.58 hr-1
D) The data is insufficient to calculate rate constant.
Correct Answer:
Verified
Q59: Hydrogen iodide decomposes at 800 K via
Q68: Given the following balanced equation,determine the rate
Q116: A particular first-order reaction has a rate
Q119: A decomposition reaction has a half-life that
Q120: Which rate law is consistent with the
Q121: What is the overall order for the
Q122: A reaction follows the following two-step mechanism.
2A
Q123: Which of the following conditions must be
Q125: The graph represents the first-order decomposition of
Q176: For the first-order reaction,2 N2O(g)→ 2 N2(g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents