Which of the following metals will dissolve in nitric acid but not hydrochloric? 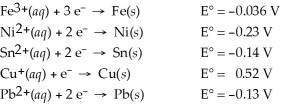
A) Fe
B) Pb
C) Cu
D) Sn
E) Ni
Correct Answer:
Verified
Q35: Which of the following reactions would have
Q50: What mass of aluminum can be plated
Q55: Calculate the cell potential for the following
Q56: Use the tabulated half-cell potentials below to
Q57: Which of the following metals will dissolve
Q58: How many electrons are transferred in the
Q61: Explain the use of a salt bridge.
Q62: Match the following.
-Q > K
A)Ecell = E°cell
B)Ecell
Q105: What is the shorthand notation that represents
Q118: For the galvanic cell reaction,expressed below using
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents