Calculate the cell potential for the following reaction that takes place in an electrochemical cell at 25°C.
Sn(s) ∣ Sn2+(aq,1.8 M) ∣∣ Ag+(aq,0.055 M) ∣ Ag(s) 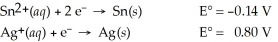
A) -0.94 V
B) -0.85 V
C) +1.02 V
D) +0.98 V
E) +0.86 V
Correct Answer:
Verified
Q38: Which of the following is the strongest
Q39: Determine which of the following pairs of
Q40: Which of the following is the strongest
Q41: Calculate the cell potential for the following
Q42: Give the criteria for a nonspontaneous reaction.
A)ΔG°
Q44: How many electrons are transferred in the
Q45: Calculate the cell potential for the following
Q46: Which of the following reactions would be
Q47: Give the criteria for a spontaneous reaction.
A)ΔG°
Q48: Use the tabulated half-cell potentials below to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents