The following reaction represents what nuclear process? 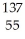 Cs +
Cs +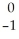 E →
E →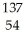 Xe
Xe
A) beta emission
B) positron emission
C) gamma emission
D) electron capture
E) alpha capture
Correct Answer:
Verified
Q34: Determine the identity of the daughter nuclide
Q35: Identify the missing particle in the following
Q35: Fluorine-18 undergoes positron emission with a half-life
Q36: Determine the identity of the daughter nuclide
Q37: Determine the identity of the daughter nuclide
Q38: Determine the identity of the daughter nuclide
Q40: Determine the identity of the daughter nuclide
Q41: Nuclides below the valley of stability can
Q43: Identify the elements used in radiometric dating.
A)Carbon-14
Q44: Determine the half-life of a nuclide that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents