The following reaction represents what nuclear process? 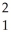 H +
H +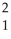 H →
H →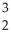 He +
He +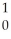 N
N
A) nuclear fusion
B) alpha emission
C) beta emission
D) nuclear fission
E) neutron capture
Correct Answer:
Verified
Q28: Calculate the mass defect in Fe-56 if
Q42: Determine the binding energy of an O-16
Q66: Why is an alpha emitter much more
Q67: Match the following. Q68: Identify the element that is not used Q69: Match the following. Q70: Explain how radiation increases cancer risk. Q76: Match the following. Q132: Explain the concept of "magic numbers." Q135: What is the "mass defect"? Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents
-positron
A)![]()
-proton
A)![]()
-gamma ray
A)![]()

