Object 1 has three times the specific heat capacity and four times the mass of Object 2.The two objects are given the same amount of heat.If the temperature of Object 1 changes by an amount ΔT,the change in temperature of Object 2 will be
A) ΔT.
B)  ΔT.
ΔT.
C) 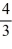 ΔT.
ΔT.
D) 6ΔT.
E) 12ΔT.
Correct Answer:
Verified
Q14: A thermally isolated system is made up
Q15: Which two temperature changes are equivalent?
A)1 K
Q16: Consider a flat steel plate with a
Q17: As shown in the figure,a bimetallic strip,consisting
Q18: If you wanted to know how much
Q20: If the absolute temperature of an object
Q21: Express -40°C in °F.
A)-72°F
B)-54°F
C)-40°F
D)4.4°F
Q22: Nitrogen boils at -196°C.What is the corresponding
Q23: A person running in place on an
Q24: The temperature changes from 35°F during the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents