A 920-g empty iron kettle is put on a stove.How much heat in joules must it absorb to raise its temperature form 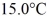
To 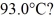
The specific heat for iron is 113 cal/kg ∙ °C,and 1 cal = 4.186 J.
A) 33,900 J
B) 40,500 J
C) 8,110 J
D) 40,100 J
Correct Answer:
Verified
Q75: On his honeymoon,James Joule attempted to explore
Q76: A camper is about to drink his
Q77: The water flowing over Niagara Falls drops
Q78: A 90-g aluminum calorimeter contains 390 g
Q79: A carpenter is driving a 15.0-g steel
Q81: A concrete wall of a cold storage
Q82: A concrete wall of a cold storage
Q83: A 0.600-kg piece of metal X is
Q84: Two metal rods,one silver and the other
Q85: The thermal conductivity of a certain concrete
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents