A glass beaker of unknown mass contains 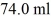
Of water.The system absorbs 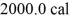
Of heat and the temperature rises 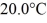
As a result.What is the mass of the beaker? The specific heat of glass is 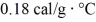
,and that of water is 1.0 cal/g ∙ °C.
A) 140 g
B) 560 g
C) 540 g
D) 270,000 g
Correct Answer:
Verified
Q57: The coefficient of linear expansion of copper
Q58: If 150 kcal of heat raises the
Q59: A brass rod is 69.5 cm long
Q60: A mercury thermometer has a glass bulb
Q61: In grinding a steel knife,the metal can
Q63: A 6.5-g iron meteor hits the earth
Q64: A lab student drops a 400.0-g piece
Q65: A 5.00-g lead BB moving at 44.0
Q66: An aluminum electric tea kettle with a
Q67: A person tries to heat up her
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents