A 90-g aluminum calorimeter contains 390 g of water at an equilibrium temperature of 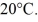
A 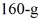
Piece of metal,initially at 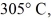
Is added to the calorimeter.The final temperature at equilibrium is 32° C.Assume there is no external heat exchange.The specific heat capacities of aluminum and water are 910 J/kg ∙ K (aluminum) and 4190 J/kg ∙ K (water) .What is the specific heat capacity of the 160-g piece of metal?
A) 470 J/kg ∙ K
B) 430 J/kg ∙ K
C) 350 J/kg ∙ K
D) 310 J/kg ∙ K
E) 510 J/kg ∙ K
Correct Answer:
Verified
Q73: A lab assistant drops a 400.0-g piece
Q74: A 200-L electric water heater uses 2.0
Q75: On his honeymoon,James Joule attempted to explore
Q76: A camper is about to drink his
Q77: The water flowing over Niagara Falls drops
Q79: A carpenter is driving a 15.0-g steel
Q80: A 920-g empty iron kettle is put
Q81: A concrete wall of a cold storage
Q82: A concrete wall of a cold storage
Q83: A 0.600-kg piece of metal X is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents