Consider two equal-volume flasks of gas at the same temperature and pressure.One gas,oxygen,has a molecular mass of 32.The other gas,nitrogen,has a molecular mass of 28.What is the ratio of the number of oxygen molecules to the number of nitrogen molecules in these flasks?
A) 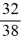
B) 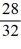
C) 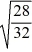
D) 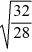
E) 
Correct Answer:
Verified
Q4: The absolute temperature of an ideal gas
Q5: Which of the following are SI units
Q6: Oxygen molecules are 16 times more massive
Q7: The absolute temperature of a gas is
Q8: A 25-kg piece of equipment can be
Q10: A fixed container holds oxygen and helium
Q11: The figure shows a graph of the
Q12: How much heat must be removed from
Q13: Oxygen molecules are 16 times more massive
Q14: Two wires are made out of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents