If you add 700 kJ of heat to 700 g of water originally at 70.0°C,how much water is left in the container? The latent heat of vaporization of water is 22.6 × 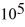
J/kg,and its specific heat capacity is 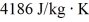
.
A) 429 g
B) 258 g
C) 340 g
D) 600 g
E) none
Correct Answer:
Verified
Q30: A 44.0-g block of ice at -15.0°C
Q31: A substance has a melting point of
Q32: A 45.0-kg sample of ice is at
Q33: A 400-g block of iron at 400°C
Q34: A substance has a melting point of
Q36: Solar houses use a variety of energy
Q37: The melting point of aluminum is 660°C,its
Q38: If you add 1.33 MJ of heat
Q39: Heat is added to a 3.0 kg
Q40: A 771.0-kg copper bar is put into
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents