What is the average translational kinetic energy of an ideal gas at 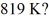
The Boltzmann constant is 1.38 × 10-23 J/K.
A) 1.70 × 10-20 J
B) 5.65 × 10-21 J
C) 1.13 × 10-17 J
D) 3.77 × 10-19 J
Correct Answer:
Verified
Q58: A laboratory vacuum pump can reduce the
Q59: How many moles are there in 2.00
Q60: A balloon originally has a volume of
Q61: The molecular weight of nitrogen,N2,is 28 g/mol.What
Q62: An oxygen molecule,O2,falls in a vacuum.From what
Q64: A 4.2-L flask of ideal neon gas
Q65: Dust particles in a grain elevator frequently
Q66: A sealed container holds 0.020 moles of
Q67: The rms speed of a certain sample
Q68: A 24.0-L tank contains ideal helium gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents