A sealed 87- 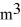
Tank is filled with 6,000 moles of ideal oxygen gas O2 at an initial temperature of 270 K.The gas is heated to a final temperature of 460 K.The atomic mass of oxygen is 16.0 g/mol.How much heat is transferred to the gas during this process? (R = 8.31 J/mol ∙ K)
A) 24 MJ
B) 28 MJ
C) 19 MJ
D) 14 MJ
E) 9.1 MJ
Correct Answer:
Verified
Q56: A heat engine having the maximum possible
Q57: Two ideal Carnot heat engines have the
Q58: An ideal Carnot heat engine operates between
Q59: The ocean thermal energy conversion project uses
Q60: The "hot shot" heat engine operating between
Q62: A container with rigid walls is filled
Q63: A Carnot air conditioner has a coefficient
Q64: How much heat is required to increase
Q65: An ideal reversible heat pump is taking
Q66: A rigid container is filled with 4.0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents