The temperature of an ideal gas in a sealed rigid 0.60- 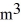
Container is reduced from 460 K to 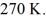
The final pressure of the gas is 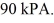
The molar heat capacity at constant volume of the gas is 28.0 J/mol ∙ K.How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)
A) -130 kJ
B) -170 kJ
C) 130 kJ
D) 170 kJ
E) 0 kJ
Correct Answer:
Verified
Q70: An ideal Carnot air conditioner operates between
Q71: The figure shows a pV diagram for
Q72: An ideal Carnot engine is operated as
Q73: An ideal Carnot refrigerator with a performance
Q74: An ideal Carnot engine is operated as
Q76: The figure shows a pV diagram for
Q77: How much heat is required to raise
Q78: A heat pump absorbs heat from the
Q79: A sample of ideal monatomic gas is
Q80: The figure shows a pV diagram for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents