The figure shows a pV diagram for 8.3 g of ideal nitrogen gas N2 in a sealed container.The temperature of state 1 is 59°C,the atomic mass of the nitrogen atom is 14 g/mol,and R = 8.31 J/mol ∙ K.What are (a) pressure p1 and (b) temperature T2? 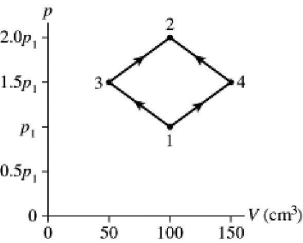
A) (a) 81 atm,(b) 660°C
B) (a) 14 atm,(b) 660°C
C) (a) 81 atm,(b) 120°C
D) (a) 14 atm,(b) 120°C
Correct Answer:
Verified
Q71: The figure shows a pV diagram for
Q72: An ideal Carnot engine is operated as
Q73: An ideal Carnot refrigerator with a performance
Q74: An ideal Carnot engine is operated as
Q75: The temperature of an ideal gas in
Q77: How much heat is required to raise
Q78: A heat pump absorbs heat from the
Q79: A sample of ideal monatomic gas is
Q80: The figure shows a pV diagram for
Q81: An ideal gas undergoes the process a→b→c→a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents