The figure shows a pV diagram for 0.0061 mol of ideal gas that undergoes the process 1 → 2 → 3.What is the pressure p2? (R = 8.31 J/mol ∙ K) 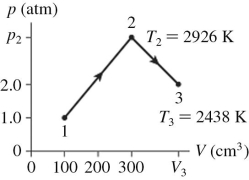
A) 4.9 atm
B) 4.9 × 105 atm
C) 15 atm
D) 1.5 × 106 atm
Correct Answer:
Verified
Q80: The figure shows a pV diagram for
Q81: An ideal gas undergoes the process a→b→c→a
Q82: A gas expands from an initial volume
Q83: The figure shows a pV diagram for
Q84: The figure shows a pV diagram for
Q85: The temperature of an ideal gas in
Q86: A compression at a constant pressure of
Q88: The figure shows a pV diagram for
Q89: An expansion process on an ideal diatomic
Q90: A gas expands from an initial volume
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents