A jeweler needs to electroplate gold,having an atomic mass of 196.97 g/mol,onto a bracelet.He knows that the charge carriers in the ionic solution are singly-ionized gold ions,Au+,and has calculated that he must deposit 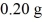
Of gold to reach the necessary thickness.How much current does he need to plate the bracelet in 3.0 hours? (e = 1.60 × 10-19 C,NA = 6.02 × 1023 atoms/mol)
A) 9.1 mA
B) 540 mA
C) 33 A
D) 1,800 mA
Correct Answer:
Verified
Q79: In an electroplating process,it is desired to
Q80: A total of 2.0 × 1013 electrons
Q81: A metal bar is 20 cm long
Q82: A 12-V battery is connected across a
Q83: A 1.0-m length of nichrome wire has
Q85: A 4000-Ω resistor is connected across a
Q86: A 1.0-mm diameter extension cord is made
Q87: A certain metal has a resistivity of
Q88: What is the resistance of 1.0 m
Q89: Calculate the current through a 10.0-m long
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents