One of the emission lines described by the original version of Balmer's formula has wavelength 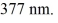
What is the value of n in Balmer's formula that gives this emission line?
A) 11
B) 12
C) 13
D) 14
Correct Answer:
Verified
Q19: The principal quantum number n can have
Q20: The Paschen series is formed by electron
Q21: The magnetic quantum number m1 can have
Q22: What value of n corresponds to a
Q23: What is the longest wavelength in the
Q25: What is the shortest wavelength in the
Q26: What is the atomic number of a
Q27: What is the longest wavelength in the
Q28: The electron spin quantum number can have
Q29: If ℓ = 4,which one of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents