The shortest wavelength of a photon that can be emitted by a hydrogen atom,for which the initial state is 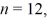
Is closest to which one of the following values? 
A) 92 nm
B) 82 nm
C) 72 nm
D) 62 nm
E) 52 nm
Correct Answer:
Verified
Q43: What is the wavelength of the photon
Q44: If light excites atomic hydrogen from its
Q45: The longest wavelength photon that can be
Q46: What is the ionization energy of the
Q47: Radio astronomers often study the radiation emitted
Q49: In a transition from one vibrational state
Q50: In a hydrogen atom,the electron makes a
Q51: A hydrogen atom is excited to the
Q52: The longest wavelength of a photon that
Q53: Light shines through atomic hydrogen gas that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents