An atom has completely filled inner shells and a single valence electron in an excited p state.The filled inner shells have an orbital momentum equal to zero.What is the magnitude of the orbital angular momentum of the atom?
A) 1.0 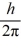
B) 1.2 
C) 1.4 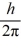
D) 1.7 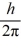
E) 2.0 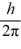
Correct Answer:
Verified
Q69: Which one of the following is the
Q70: What is the electron configuration for Li,which
Q71: Which one of the following is the
Q72: A hydrogen atom is in the 6h
Q73: In the n = 1 state,the energy
Q75: Given that the speed of an electron
Q76: What is the correct ground state electron
Q77: A hydrogen atom with a barely bound
Q78: A hydrogen atom is in the 6h
Q79: The wavelength of the emitted photon if
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents