An yttrium isotope has 39 protons and 50 neutrons in its nucleus.Which one of the following symbols accurately represents this isotope?
A) 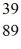 Y
Y
B) 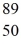 Y
Y
C) 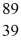 Y
Y
D) 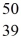 Y
Y
E) 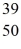 Y
Y
Correct Answer:
Verified
Q14: In a Q15: The primary reason that very large nuclei Q17: The atomic number of an atom is Q18: The binding energy per nucleon of a Q20: Which of the following statements is not Q21: During β- decay Q22: In β- decay,the number of protons in Q23: A radioactive isotope of atomic number Z Q24: The nuclei of Q40: A fusion reaction releases energy because the![]()
A)a neutron is transformed to![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents