The stability of 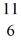
C with respect to alpha,beta-plus and beta-minus decay is to be determined.The following atomic masses are known: 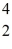
He: 4.002603 u 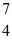
Be: 7.016928 u 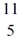
B: 11.009305 u 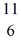
C: 11.011433 u 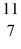
N: 11.026742 u
The 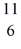
C nucleus is
A) not subject to alpha,beta-plus or beta-minus decay.
B) subject to alpha decay only.
C) subject to beta-plus decay only.
D) subject to beta-minus decay only.
E) subject to beta-plus or beta-minus decay,but not to alpha decay.
Correct Answer:
Verified
Q75: A carbon-14 nucleus decays to a nitrogen-14
Q76: The neutral helium atom, Q77: A summary of the nuclear reactions that Q78: Which of the following particles (or groups Q79: When a stationary plutonium-239 nucleus decays into Q81: How long will it take a 4.50-kBq Q82: The isotope Q83: The half-life of radon-222 is 3.83 d.If Q84: A radioactive substance with a half-life of Q85: An oxygen-15 nucleus, Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

