The stability of 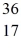
Cl with respect to alpha,beta-plus and beta-minus decay is to be determined.The following atomic masses are known: 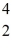
He: 4.002603 u 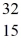
P: 31.973907 u 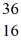
S: 35.967081 u 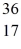
Cl: 35.968307 u 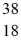
Ar: 35.967546 u
The 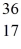
Cl nucleus is
A) not subject to alpha,beta-plus or beta-minus decay.
B) subject to alpha decay only.
C) subject to beta-plus decay only.
D) subject to beta-minus decay only.
E) subject to beta-plus or beta-minus decay,but not to alpha decay.
Correct Answer:
Verified
Q62: Which of the following particles are not
Q63: A beryllium-8 nucleus at rest undergoes double
Q64: One of the fusion reactions that occurs
Q65: How many quarks are in a deuteron,
Q66: The diameter of an atom is 1.4
Q68: When a stationary plutonium-239, Q69: The neutral deuterium atom, Q70: Uranium-238 decays into thorium-234 plus an alpha Q71: How many quarks are in a tritium Q72: The following masses are known: ![]()
![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents