Radium-226 decays into radon-222 plus an alpha particle.The relevant masses are 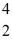
He: 4.002603 u 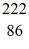
Rn: 222.017570 u 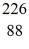
Ra: 226.025402 u
How much energy is released in this process? (1 u = 931.5 MeV/c2)
A) 4.24 MeV
B) 3.76 MeV
C) 4.87 MeV
D) 5.05 MeV
E) 5.39 MeV
Correct Answer:
Verified
Q68: When a stationary plutonium-239, Q69: The neutral deuterium atom, Q70: Uranium-238 decays into thorium-234 plus an alpha Q71: How many quarks are in a tritium Q72: The following masses are known: Q74: Bismuth Q75: A carbon-14 nucleus decays to a nitrogen-14 Q76: The neutral helium atom, Q77: A summary of the nuclear reactions that Q78: Which of the following particles (or groups Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()

![]()
![]()

