A 0.25 M aqueous solution of potassium chloride,KCl,is tested for conductivity using the type of apparatus shown.What do you predict will happen? 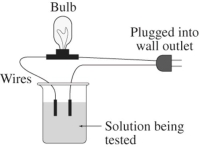
A) The bulb will not light up.KCl is a nonelectrolyte.
B) The bulb will not light up.KCl is in the molecular form when dissolved in water.
C) The light bulb will shine dimly.KCl is only partially ionized in aqueous solution.
D) The light bulb will shine brightly.KCl is highly ionized in aqueous solution.
Correct Answer:
Verified
Q32: The numbers 1 through 4 are used
Q34: Which is the correct formula (including charge)of
Q36: Which corresponds to the composition of the
Q36: When the _ molecules of ethanol,C2H5OH,are added
Q38: What is the formula of the ionic
Q39: How many joules are required to heat
Q40: Which is not a consequence of hydrogen
Q40: Which corresponds to the composition of the
Q41: Which of the following is/are true about
Q42: Which is not a form of chlorine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents