A 0.25 M solution of the sugar sucrose,C12H22O11,in water is tested for conductivity using the type of apparatus shown.What do you predict will happen? 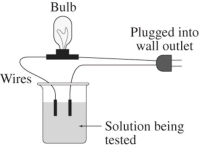
A) The bulb will not light up.Sucrose is an electrolyte,but not very soluble in aqueous solution.
B) The bulb will not light up.Sucrose is in the molecular form in aqueous solution.
C) The light bulb will shine dimly.Sucrose is only partially ionized in aqueous solution.
D) The light bulb will shine brightly.Sucrose is highly ionized in aqueous solution.
Correct Answer:
Verified
Q6: In general,hydrogen bonds are
A)about ten times stronger
Q16: Generally,the water that has the least harmful
Q17: Which correctly describes groundwater?
A)any water found above
Q18: A 4-L sample of water contains 80
Q20: In general,hydrogen bonds are _ and _
Q22: Which best symbolizes the hydrogen bonding between
Q23: What is the correct name for (NH4)2CO3?
A)biammonium
Q24: Which shows the Lewis structure of water
Q24: Which statement is not true?
A)In forming a
Q26: Which is the best representation of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents