Which compounds are not soluble in water at room temperature? 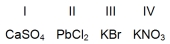
A) I and II only
B) II and III only
C) III and IV only
D) I and IV only
Correct Answer:
Verified
Q23: The main reason that water supplies are
Q51: Reverse osmosis is illustrated in this diagram.What
Q52: Which compound is insoluble in water?
A)sodium carbonate
B)potassium
Q53: Which form of water disinfection continues to
Q54: We cannot effectively clean nonpolar substances from
Q56: The steps in the typical purification processes
Q57: Which compound should be the most soluble
Q58: Which statement is not correct?
A)Lead has been
Q59: What are the major disadvantages of using
Q60: A disadvantage of ozonation over chlorination is
A)the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents