A sample of an ideal gas is heated and its Kelvin temperature doubles. What happens to the average speed of the molecules in the sample?
A) It does not change.
B) It halves.
C) It doubles.
D) It is 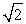 times as large.
times as large.
E) It is 1/ 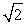 times smaller.
times smaller.
Correct Answer:
Verified
Q9: When a vapor condenses,
A) the temperature of
Q10: When a solid melts,
A) the temperature of
Q25: A liquid boils when its vapor pressure
A)
Q27: Steam burns can be much more severe
Q28: Which of the following best explains why
Q29: The heat required to change a substance
Q31: When we say that a material sample
Q32: When a liquid evaporates
A) the temperature of
Q34: When a liquid freezes
A) the temperature of
Q35: In a city high in the mountains,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents