Two containers of equal volume each hold samples of the same ideal gas. Container A has twice as many molecules as container B. If the gas pressure is the same in the two containers, the correct statement regarding the absolute temperatures TA and TB in containers A and B, respectively, is
A) TA = TB .
B) TA = 2TB .
C) TA = 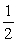 TB .
TB .
D) TA = 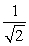 TB .
TB .
E) TA = 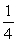 TB .
TB .
Correct Answer:
Verified
Q9: When a vapor condenses,
A) the temperature of
Q19: When a spray can is being used,
Q20: The internal energy of an ideal gas
Q21: The point in the phase diagram where
Q22: The point in the phase diagram where
Q23: A sample of an ideal gas is
Q25: A liquid boils when its vapor pressure
A)
Q27: Steam burns can be much more severe
Q28: Which of the following best explains why
Q29: The heat required to change a substance
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents