FIGURE 18-1 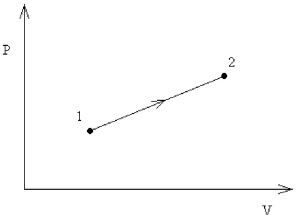
-An ideal monatomic gas undergoes the reversible expansion shown in Figure 18-1, where V2 = 5V1 and P2 = 3P1. What is the change in internal energy of the gas in this process, in terms of the initial pressure and volume?
A) 7 P1V1
B) 14 P1V1
C) 21 P1V1
D) 15 P1V1
E) 29 P1V1
Correct Answer:
Verified
Q20: A Diesel engine uses adiabatic heating to
Q21: For an ideal monatomic gas,
A) Cp =
Q22: FIGURE 18-1 Q23: A monatomic ideal gas is compressed isothermically Q24: The molar specific heat is the amount Q26: When objects at different temperatures are brought Q27: In a given reversible process, the temperature Q28: When a gas expands adiabatically, Q29: From the following statements regarding the ratio Q30: An ideal monatomic gas undergoes a reversible![]()
A) the internal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents