FIGURE 18-3 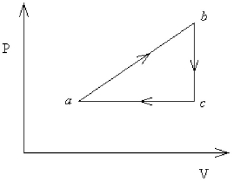
-An ideal gas undergoes the process a→b→c→a shown in Figure 18-3. The heat gained in process a→b is 546 J, while in process b→c the system loses 62 J. In process a→b the system performs 310 J of work, while in process c→a work is done on the system in the amount of 223 J. How much heat is gained by the system in process c→a?
A) - 397 J
B) - 62 J
C) 223 J
D) 18 J
E) - 236 J
Correct Answer:
Verified
Q43: What is the change in entropy of
Q44: FIGURE 18-3 Q45: A heat engine absorbs 64 kcal of Q46: Which of the following is a statement Q47: FIGURE 18-3 Q49: One of the most efficient engines built Q50: A reversible engine takes in 555. Joules Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()

