Plutonium-239 decays into uranium-235 plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values: 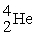 , 4.002603 u;
, 4.002603 u; 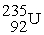 , 235.043924 u; what is the mass value of
, 235.043924 u; what is the mass value of 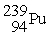 ?
?
1 u = 931.494 MeV/c2.
A) 239.052157 u
B) 239.027750 u
C) 239.001889 u
D) 238.9991889 u
E) 238.988842 u
Correct Answer:
Verified
Q70: An electron and a positron annihilate each
Q71: Uranium-238 decays into thorium-234 plus an alpha
Q72: The decay constant of radon is 0.181
Q73: Americium-243 has a decay constant of 9.39
Q74: Radium-226 decays into radon-222 plus an alpha
Q76: Carbon-14 has a half-life of 5730 years.
Q77: Fermium-253 has a half-life of 3.00 days.
Q78: An oxygen-15 nucleus ( Q79: A stationary plutonium-239 nucleus decays into a Q80: Rutherfordium-261 has a half-life of 1.08 min.![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents