Bromine 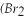 )has a freezing point of -7 °C,and a boiling point of 60 °C.
)has a freezing point of -7 °C,and a boiling point of 60 °C.
Indicate the state present or the change of state occurring at each temperature.
--7 °C
Correct Answer:
Verified
Q69: The specific heat of water (in J/g
Q73: A temperature of 20°C is equivalent to
Q80: On a heating curve a plateau corresponds
Q83: Water vapor is a gas.
Q90: One gram of fat has less energy
Q93: To calculate the Kelvin temperature,subtract 273 from
Q94: A liquid has its own volume and
Q100: Bromine ( Br2 )
-Air is a heterogeneous
Q103: The specific heat has units of kcal.
Q105: When a liquid is boiling,its temperature does
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents