Multiple Choice
The correct formula for the compound formed from Mg and S is ________.
A) MgS
B) 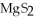
C) 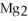 S
S
D) 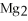
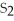
E) 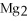
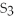
Correct Answer:
Verified
Related Questions
Q2: An ionic compound _.
A)has a net positive
Q3: In ionic compounds,_ lose their valence electrons
Q3: Which one of the following compounds contains
Q5: The compound Q6: What is the ionic charge of an Q8: The ion of aluminum is _. Q9: An anion always _. Q9: What is the symbol for the ion Q10: What is the correct formula for the Q11: The name of the ![]()
A)
A)has a positive charge
B)contains![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents